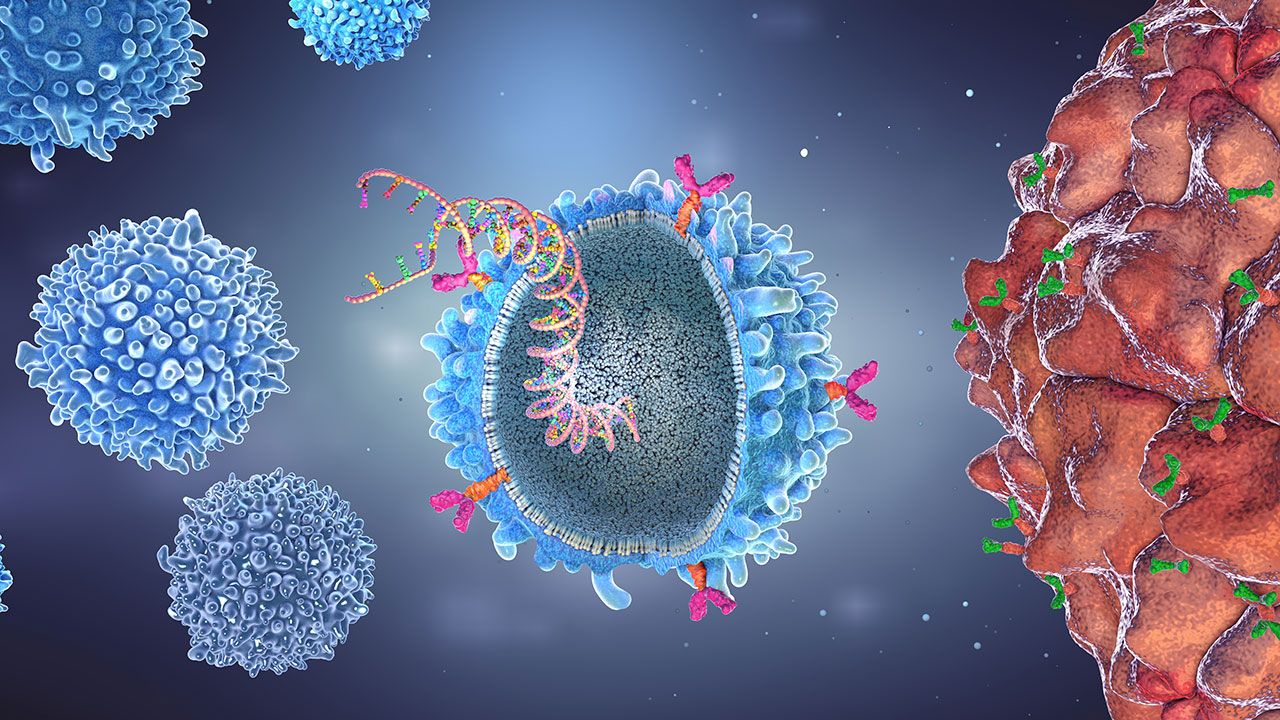
Where is CAR-T Cell Therapy Approved?
CAR-T cells are being extensively investigated in both hematological and solid malignancies. However, they are currently approved for Acute Lymphoblastic Leukemia (ALL), Lymphomas, and Multiple Myeloma. It is important to note that CAR-T cells are not yet approved for first-line management of these malignancies. They are used for patients who are relapsed and refractory, who have exhausted other therapeutic options, including bone marrow transplantation.
What Are the Side Effects of CAR-T Cell Therapy?
CAR-T cells, being genetically modified, come with side effects. Commonly expected side effects include cytokine release syndrome, neutropenia, infections, and, rarely, neurologic sequelae. These side effects can be managed with timely intervention.
Can CAR-T Cell Therapy Cure Cancer?
Evidence suggests that CAR-T cells are very effective in eradicating cancer in many patients, with benefits potentially lasting for many years, even after multiple lines of treatment. In the future, CAR-T cell therapy might become more curative when used earlier in treatment.
How Is CAR-T Therapy Different from Bone Marrow Transplantation?
In Bone Marrow Transplantation (BMT), stem cells are collected from the patient's blood or a relative's blood and infused into the patient after high-dose chemotherapy. Chemotherapy helps kill cancer, and BMT aids in the recovery of blood cells. In contrast, CAR-T cell therapy involves collecting T cells from the patient when their disease is in remission, genetically modifying these T cells to target cancer cell antigens, and then infusing them back into the patient to kill cancer cells.